Oct 25, 2023Formaldehyde, acetaldehyde, and acetone are ranked based on their electrophile strength as: Formaldehyde is the strongest followed by acetaldehyde, with acetone being the weakest. Explanation: Your question suggests to rank acetone, acetaldehyde, and formaldehyde in order of decreasing electrophile strength.
NCERT CBSE Standard 11 Chemistry Chapter 13 Hydrocarbons SKMClasses Bangalore Subhashish Sir | SKM Classes Bangalore
Chemistry Chemistry questions and answers Rank the structures in order of decreasing electrophilic strength. Most electrophilic Least electrophilic Answer Bank 人人人人 OH CI NH2 This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
Source Image: quora.com
Download Image
Concept explainers Question Can I have help with this ranking? Transcribed Image Text: Rank the structures in order of decreasing electrophilic strength. Most electrophilic CI *NH2 ОН Least electrophilic Answer Bank Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps with 1 images See solution

Source Image: youtube.com
Download Image
Answered: Rank the structures in order of… | bartleby Most electrophilic Least electrophilic Answer Bank EN NH2 HO. Rank the structures in order of decreasing electrophilic strength. Most electrophilic Least electrophilic Answer Bank EN NH2 HO. BUY. Organic Chemistry. 9th Edition. ISBN: 9781305080485. Author: John E. McMurry. Publisher: Cengage Learning.

Source Image: chemistrynotmystery.com
Download Image
Rank The Structures In Order Of Decreasing Electrophile Strength.
Most electrophilic Least electrophilic Answer Bank EN NH2 HO. Rank the structures in order of decreasing electrophilic strength. Most electrophilic Least electrophilic Answer Bank EN NH2 HO. BUY. Organic Chemistry. 9th Edition. ISBN: 9781305080485. Author: John E. McMurry. Publisher: Cengage Learning. VIDEO ANSWER: In order for hydrogen to act as a protons, we need to arrange them in the correct order so that the first one is a protons with no electrons and the second one is a hydrogen with no electrons.
Chemistry!!! Not Mystery
Electrophiles. In the vast majority of the nucleophilic substitution reactions you will see in this and other organic chemistry texts, the electrophilic atom is a carbon which is bonded to an electronegative atom, usually oxygen, nitrogen, sulfur, or a halogen. The concept of electrophilicity is relatively simple: an electron-poor atom is an Elucidation of Catalytic Propane Dehydrogenation Using Theoretical and Experimental Approaches: Advances and Outlook | Energy & Fuels

Source Image: pubs.acs.org
Download Image
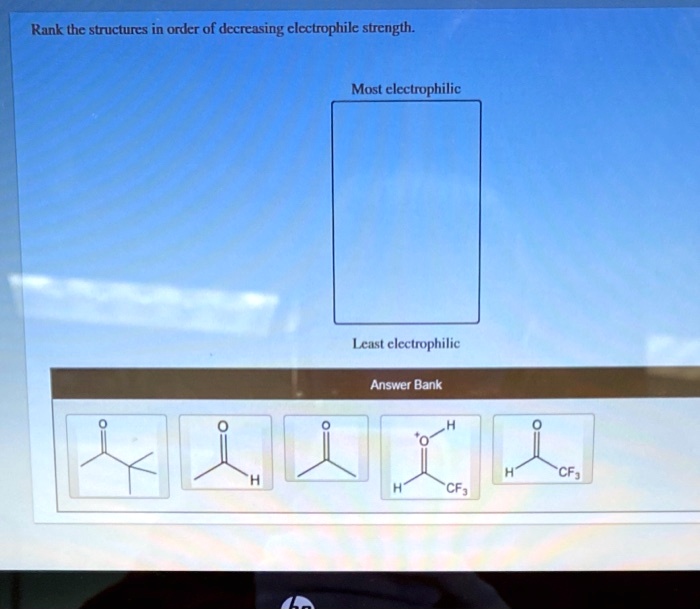
SOLVED: Rank the structures in order of decreasing electrophile strength: Most electrophilic Least electrophilic Answer Bank CF3 CF3 Electrophiles. In the vast majority of the nucleophilic substitution reactions you will see in this and other organic chemistry texts, the electrophilic atom is a carbon which is bonded to an electronegative atom, usually oxygen, nitrogen, sulfur, or a halogen. The concept of electrophilicity is relatively simple: an electron-poor atom is an

Source Image: numerade.com
Download Image
NCERT CBSE Standard 11 Chemistry Chapter 13 Hydrocarbons SKMClasses Bangalore Subhashish Sir | SKM Classes Bangalore Oct 25, 2023Formaldehyde, acetaldehyde, and acetone are ranked based on their electrophile strength as: Formaldehyde is the strongest followed by acetaldehyde, with acetone being the weakest. Explanation: Your question suggests to rank acetone, acetaldehyde, and formaldehyde in order of decreasing electrophile strength.

Source Image: zookeepersblog.wordpress.com
Download Image
Answered: Rank the structures in order of… | bartleby Concept explainers Question Can I have help with this ranking? Transcribed Image Text: Rank the structures in order of decreasing electrophilic strength. Most electrophilic CI *NH2 ОН Least electrophilic Answer Bank Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps with 1 images See solution

Source Image: bartleby.com
Download Image
Chemistry!!! Not Mystery Rank the following structures in order of decreasing electrophile strength. most electrophilic least electrophilic CF3 `CF3 Н Organic Chemistry: A Guided Inquiry 2nd Edition ISBN: 9780618974122 Author: Andrei Straumanis Publisher: Andrei Straumanis Chapter8: Addition Via Carbocation Section: Chapter Questions Problem 8E See similar textbooks

Source Image: chemistrynotmystery.com
Download Image
SOLVED: Rank the structures in order of decreasing electrophile strength: Most electrophilic Least electrophilic Answer Bank CF3 CF3 Most electrophilic Least electrophilic Answer Bank EN NH2 HO. Rank the structures in order of decreasing electrophilic strength. Most electrophilic Least electrophilic Answer Bank EN NH2 HO. BUY. Organic Chemistry. 9th Edition. ISBN: 9781305080485. Author: John E. McMurry. Publisher: Cengage Learning.
Source Image: numerade.com
Download Image
Rank the aromatics shown in order of decreasing reactivity toward electrophilic substitution (more reactive > least reactive) VIDEO ANSWER: In order for hydrogen to act as a protons, we need to arrange them in the correct order so that the first one is a protons with no electrons and the second one is a hydrogen with no electrons.
 Download Image
Download ImageSOLVED: Rank the structures in order of decreasing electrophile strength: Most electrophilic Least electrophilic Answer Bank CF3 CF3
Rank the aromatics shown in order of decreasing reactivity toward electrophilic substitution (more reactive > least reactive) Chemistry Chemistry questions and answers Rank the structures in order of decreasing electrophilic strength. Most electrophilic Least electrophilic Answer Bank 人人人人 OH CI NH2 This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
Answered: Rank the structures in order of… | bartleby SOLVED: Rank the structures in order of decreasing electrophile strength: Most electrophilic Least electrophilic Answer Bank CF3 CF3 Rank the following structures in order of decreasing electrophile strength. most electrophilic least electrophilic CF3 `CF3 Н Organic Chemistry: A Guided Inquiry 2nd Edition ISBN: 9780618974122 Author: Andrei Straumanis Publisher: Andrei Straumanis Chapter8: Addition Via Carbocation Section: Chapter Questions Problem 8E See similar textbooks